Exploring Key Differences and Market Impact
With demand for electric vehicles on the rise, the demand for lithium-ion batteries and the materials that make them function has also skyrocketed. Graphite is a key ingredient in these batteries for storing energy. But did you know there are two types of graphite that can be used: natural and synthetic, also known as engineered graphite? Understanding the differences between them is crucial for figuring out their roles in the battery market.
The future of natural graphite hinges on its ability to consistently meet high quality standards. Stephen Riddle, former CEO and current advisor at Asbury Carbon, a graphite expert with over 45 years of experience, emphasises that maintaining these standards is critical for natural graphite to compete effectively with synthetic/engineered graphite.
Graphitic Structure and Energy Storage Capability
Graphite’s structure is what makes it so effective in batteries. It has a layered design, which allows lithium ions to slip between these layers when the battery is charged, storing energy for later use when the device needs power.
Natural graphite, with its complex and varied structure, possesses higher crystallinity. This feature enables it to store more ions and conduct energy more effectively, leading to enhanced energy storage and improved battery performance, particularly in applications requiring high energy density.
Synthetic graphite is made by heating petroleum coke or coal tar to very high temperatures, creating a uniform carbon structure ideal for consistent battery performance. However, the process is energy-intensive, making synthetic graphite costly to produce and less environmentally friendly. These high energy demands limit its use, especially in regions with strict environmental regulations or high energy costs.
Production and Processing: Purification and Spheronization
The process of making natural graphite suitable for batteries involves several steps. After mining, the graphite needs to be purified to reach a carbon content of over 99.95%. Techniques like acid leaching or caustic purification help remove impurities, though some methods such as using hydrofluoric acid, raise environmental and safety concerns. More sustainable methods, like using sulfuric acid offer cleaner alternatives for purification.
In addition, natural graphite undergoes spheronization, a process that shapes the particles into spheres. This maximizes the material’s tap density, boosting the battery’s energy storage capacity, an essential step for use in high-performance applications like electric vehicles.
Surface Treatment and Solid Electrolyte Interphase Formation
To get the most out of both natural and synthetic graphite, manufacturers often apply carbon coatings to their surfaces. This helps prevent unwanted reactions between the graphite and the battery’s electrolyte, which can form something called the Solid Electrolyte Interphase (SEI). The SEI is necessary for battery function, but it can degrade over time, leading to shorter battery life.
Natural graphite, with its impurities, is more likely to form an unstable SEI, making it harder to maintain. Synthetic graphite, being more uniform, has fewer issues here, but coating it still helps keep the SEI stable, ensuring the battery lasts longer and performs better.
Comparing Performance
Synthetic graphite is often preferred for its consistent performance and durability, as it can handle more charge/discharge cycles without degrading as quickly. It also avoids some of the swelling issues that can affect natural graphite due to its more varied structure. However, synthetic graphite is much more expensive to produce, making it less practical for widespread use in lower-cost applications.
On the other hand, natural graphite is more affordable and increasingly popular with battery manufacturers, especially for its environmental benefits and scalability. One of the biggest risks for natural graphite suppliers is related to consistency and quality. For natural graphite to become a viable alternative to synthetic graphite, it must meet strict performance standards, especially for high-tech applications like lithium-ion batteries. As Stephen Riddle points out, if natural graphite producers can prove they have a consistent and reliable process, the adoption of natural graphite anodes will likely increase. However, to match the durability and stability of synthetic graphite, natural graphite typically needs more advanced processing and surface treatments in high-performance applications.
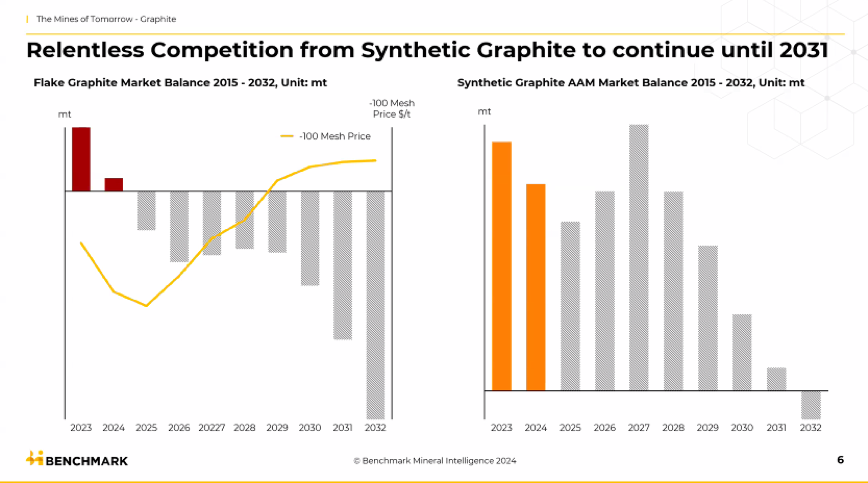
Environmental and Cost Considerations
The environmental impact of synthetic graphite production is a major concern due to the energy-intensive graphitization process. As the global push for sustainable supply chains intensifies, many automakers and battery producers are seeking alternatives.
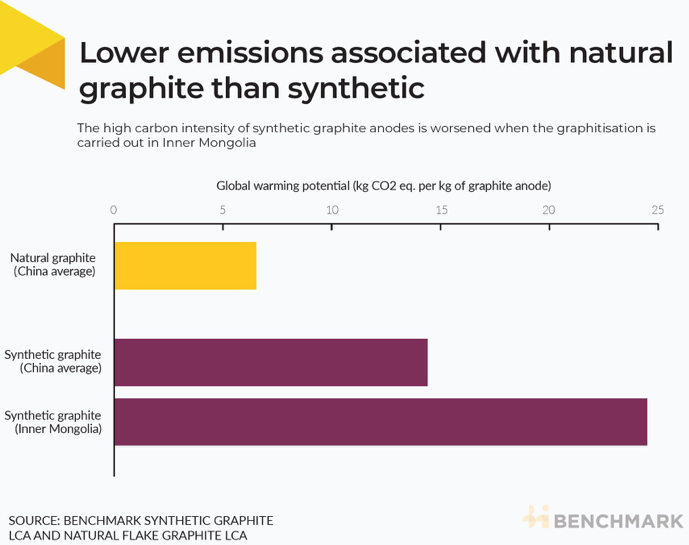
According to Benchmark’s Life Cycle Assessments (LCA), the production of natural graphite anodes results in approximately 55% less carbon emissions than synthetic graphite anodes produced in China. This highlights natural graphite’s environmental advantages, particularly in terms of sustainability.
Even though natural graphite requires additional purification steps, it’s generally still more affordable than synthetic. With EV production ramping up, this cost advantage could make natural graphite the go-to choice.
Market Impact and Future Trends
The global graphite market is set to experience a significant supply-demand imbalance in the future, driven by the rapid growth of the EV market. Natural graphite is expected to play a central role in meeting this demand, especially as new battery technologies emerge that favor ESG compliant materials.
While several projects outside China, including those in Africa, Australia, and the Americas, show promise, they are still largely in the early stages of either development or production. These emerging operations face significant risks, from unexpected delays to challenges in meeting the high purity and processing standards required by the battery industry – issues that ultimately impact profitability. As Stephen Riddle has noted, this uncertainty creates a cautious environment for companies seeking reliable graphite supply. Although there is a strong desire to diversify away from China, many companies may still turn to Chinese suppliers in the short term. China’s well-established graphite industry, with proven capabilities in both mining and downstream processing, makes it difficult for new entrants to compete effectively right away. But how long can the reliance on China last?
Conclusion: A Balancing Act Between Performance and Sustainability
While synthetic graphite remains the top choice for high-performance applications, natural graphite is quickly catching up. Advances in purification and spheronization are making natural graphite more competitive, especially in large-scale battery production. As sustainability becomes a bigger factor, natural graphite’s lower cost and smaller environmental impact may give it an edge.
In the near future, both natural and synthetic graphite will continue to play important roles in EV batteries. But as the world pushes for greener tech, natural graphite could become the more popular option.
